Olavo T. Fontès : Flying Saucers: The Startling Evidence Of The Invasion From Outer Space de Coral Lorenzen (1966),
: Flying Saucers: The Startling Evidence Of The Invasion From Outer Space de Coral Lorenzen (1966),
 : Flying Saucers: The Startling Evidence Of The Invasion From Outer Space de Coral Lorenzen (1966),
: Flying Saucers: The Startling Evidence Of The Invasion From Outer Space de Coral Lorenzen (1966), It is widely known that since 1947 many people in many places have reported "flying saucers" and other strange objects in the sky. But the absence of physical evidence such as crashed "saucers" has been considered the best argument against the existence of such UFOs. In fact, it is difficult to recognize the reality of a flying machine so far advanced as to have reduced to near zero the probability of mechanical failure. Major Edward J. Ruppelt, USAFR, in his excellent book, A Report on Unidentified Flying Objects, states that the USAF had never picked up any "hardware" whole "saucers," pieces or parts that could not be readily identified as something very earthly.
Such an unexpected occurrence was reported, at last, near the Brazilian coast. It was said a disc shaped object had exploded over the seashore. Fragments recovered from the explosion were supposed to have fallen, while burning, into shallow waters, which, according to the witnesses, quenched the fire and allowed recovery. I cannot vouch for the story, but only for the identity of the samples received and the details of the investigation that followed. The story of the origin of the samples will be of interest in connection with the results of the chemical analyses which were performed.
On September 14, 1957, Ibrahim Sued, a well known Rio Janeiro society columnist, reported a strange story which startled the readers of his column in the newspaper 0 Globo Under the heading, "A Fragment From a Flying Disc,'' he wrote:
We received the letter: "Dear Mr. Ibrahim Sued. As a faithful reader of your column and your admirer, I wish to give you something of the highest interest to a newspaperman, about the flying discs. If you believe that they are real, of course. I didn't believe anything said or published about them. But just a few days ago I was forced to change my mind. I was fishing together with some friends, at a place close to the town of Ubatuba, Sao Paulo, when I sighted a flying disc. It approached the beach at unbelievable speed and an accident, i.e. a crash into the sea, seemed imminent. At the last moment, however, when it was almost striking the waters, it made a sharp turn upward and climbed rapidly on a fantastic impulse. We followed the spectacle with our eyes, startled, when we saw the disc explode in flames. It disintegrated into thousands of fiery fragments, which fell sparkling with magnificent brightness. They looked like fireworks, despite the time of the accident, at noon, i.e. at midday. Most of these fragments, almost all, fell into the sea. But a number of small pieces fell close to the beach and we picked up a large amount of this material which was as light as paper. I am enclosing a small sample of it. I don't know anyone that could be trusted to whom I might send it for analysis. I never read about a flying disc being found, or about fragments or parts of a saucer that had been picked up. Unless the finding was made by military authorities and the whole thing kept as a top secret subject. I am certain the matter will be of great interest to the brilliant columnist and I am sending two copies of this letter to the newspaper and to your home address."
From the admirer (the signature was not legible), together with the above letter, I received fragments of a strange metal...
The unusual story stirred my curiosity. Ibrahim Sued had never written about UFOs before. My first thought was the whole thing could be a joke or well planned hoax. I tried to convince myself this was the obvious explanation, and to dismiss the matter, but I felt something should be done to clarity the doubts raised in my mind. I had to contact Mr. Sued to take a look at the "fragments" and find the answer I was looking for. I phoned him that same day and ask for a meeting to discuss the matter. He agreed. I arrived at his apartment four hours later. There on the table I saw the samples sent by the unidentified correspondent three small pieces of dull gray solid substance that appeared to be a metal of some sort. Their surfaces were not smooth and polished, but quite irregular and apparently strongly oxidized. Their appearance suggested they might be, if really metallic, pieces or fragments disintegrated from a larger metallic mass or object; in fact, the surface of one of the samples was shot through with almost microscopic cracks, always longitudinal, and even showed on one face a large longitudinal fissure running through almost two thirds of its length, as if that piece had been disrupted under the action of some force. The others did not show many cracks or fissures, but the surfaces of all samples were covered in scattered areas with a whitish material. These whitish smears of a powdered substance appeared as a thin layer. The fine, dry powder was adherent, but could be displaced easily with the nail. It also filled the fissures and cracks on the surface of the first sample. This powder presented some similarity with the whitish powdered cinders on a chunk of burned charcoal as if the fragments had been scorched by some fire or were damaged by too much heat. Two of these samples were later photographed still in their original form.
Mr. Sued said the material appeared to be lead at first sight because of the gray color but I would see it could not be lead, a heavy metal, if I felt the weight of the sample in my hands. He was right. The material was light, definitely lighter than aluminum almost as light as paper. Amazed, I told Mr. Sued I had some friends with scientific backgrounds who might be called in to investigate the samples He said he knew nothing about UFOs and was even convinced they did not exist. He was not curious about the samples and I could take them. Of course, he would like to know the results if something unusual was found in the analysis. I thanked him for his generous attitude, promising to keep him informed, and picked up the samples.
On examining the data I concluded they offered insufficient solid information for a definite conclusion. A few points however, attracted my interest:
These reasons explain my interest in obtaining the samples and making a scientific investigation of the material.
The peculiar appearance of the metallic samples (if they were really metallic) indicated they could well be "fragments" originating from the explosion of a larger metallic mass or object, and that they had been burned or scorched by some kind of fire or heat. I decided to enlist the help of chemists of considerable repute. The peculiarities of the material, as well as its obvious light density, constituted a real puzzle that only scientific investigation might solve. I kept the samples for seven days before reaching my decision to send the material to a highly qualified laboratory, one of the best in my country.
The samples (the fragments it was claimed originated in the "explosion" of the reported UFO) were turned over to the Mineral Production Laboratory, a division of the National Department of Mineral Production in the Agriculture Ministry of Brazil. The laboratory is the official Brazilian institution for the examination and analysis of mineral substances, metallic ores, metals and alloys. The samples were registered there as being of "unknown origin" and were delivered personally to Dr. Feigl, the chief chemist. I was introduced to him by a friend, Dr. Julio de Morais. I hoped this famous German chemist would conduct the investigation. However, he was doing experimental studies in organic chemistry and researches on plastics at the time, and he could not make the investigation personally. He called one of his assistants, Dr. David Goldscheim, who made a careful examination of the samples and suggested their physical appearance indicated they might be fragments of meteoric origin But Dr. Feigl refused to accept such a possibility. "They are too light to be fragments of a meteorite," he said. "They appear to be metallic, made of a lightweight metal. But this metal is not aluminum. I am going to make a chemical test ...
A small chip of the material was placed in a test tube. A few drops of phosphomolybdic acid were added, plus a few drops of dilute hydrochloric acid a qualitative screening test to identify metals. If the material was metallic a blue color would appear in the test tube (phosphomolybdic acid is easily reduced, in the presence of a reducing agent, to produce the blue colored mixture of colloidal reduced oxides of molybdenum). No change was detected at first; but when the test tube was slightly heated, bubbles appeared on the surface of the material and the blue color was observed. Thus the material (or part of it) was really a metal of some sort.
It was decided that a spectrographic analysis should be made for the identification of the unknown metal in the sample, and to establish the presence of other possible constituents. The spectrographic method is extremely sensitive, making it possible to determine the chemical composition of a piece of metal no larger than the head of a pin. Minute traces of elements can be detected, traces so small they could not possibly be detected by any other known means. Each metal (as well as gasses and a few non metals) has a spectrum which is uniquely its own, whether it consists of two lines (sodium) or thousands of lines (iron), and whether the element is alone or in combination. Each element, when excited under proper conditions, gives off its spectrum; and all compounds are resolved into their components.
One of the "disc's" fragments (referred to as Sample 1) was preliminarily divided in several pieces. Two of these metallic pieces weighing approximately 0.6 grams each, were sent that same day to the Spectrographic Section of the Mineral Production Laboratory. The others were returned to me, to be kept for other analyses, if necessary. The remaining two "disc's fragments," still in my hands, were also set apart for any future investigation. These were later photographed. Unfortunately, no photograph was taken of Sample I in its original form; this was a real oversight.
The large sample (Sample 2) showed clearly the longitudinal fissure and small cracks described previously. The smaller one (Sample 3), which also presented a few small fissures, had a peculiar curved cross section. This unusual shape might suggest it came from a curved shell, a spheroid object or a dome shaped device, but in view of the heat required for oxidation it may not be significant. Both samples presented a quite irregular and apparently strongly oxidized surface. Their dull gray color contrasted with the whitish areas covered with the powdered material already described. This material was presumed to be an oxide of the metal in the samples, possibly formed when the samples were at ignition temperature and exposed to air.
I was curious about the results of the spectrographic analysis. I knew the presence or absence in the unknown material ta of any of the seventy chemical elements would be revealed by the spectrograph, and no element could be missed if it m constituted as much as the one millionth part of the whole, n It was planned that the material should be investigated by c other methods, if necessary: (1) a standard "semi quantitative'' spectrographic analysis; (2) an X ray diffraction analysis; and (3) a special "mass spectrograph" analysis.
The official analysis of the two metallic pieces taken from Sample 1 was made on September 24, 1957, by the chief chemist of the Spectrographic Section of the Mineral Production Laboratory, Dr. Luisa Maria A. Barbosa. A routine exposure was made initially, to identify the metal in the a sample. One of the metallic pieces was burned in an arc between standard electrodes in an exposure of fixed length. The metal in the sample was identified as magnesium. Then a second exposure, using the other metallic piece, was made, to determine the purity of the metal and to detect other possible elements present in the sample. This exposure was made by a special method prescribed for highly sensitive analyses using a large Hilger spectrograph for more precise and reliable results. The official report on this spectrographic analysis, signed by Dr. Barbosa, was received a few days later. (Figure IA; English translation, Figure 1B). The conclusion was that the magnesium in the sample was of unusual purity with no detectable inclusion of other elements. But since I expected a more detailed description of the results of the analysis, went to the laboratory on September 30, 1957, to meet Dr. Barbosa and request additional explanation. I tried to impress upon her the necessity of a more detailed report including technical data on the spectrum lines recorded on the photographic plate. We talked for almost an hour. She said I had no authority to appraise her work, if I were a chemist I would be satisfied with her report, etc. I tried to con her but she refused to consider my request for an additional report. In the end I asked some questions about the terpretation of the spectrographic data. Here is a summary of the questions and answers:
Did your analysis show the presence of magnesium of unusual purity and absence of any other metallic element.
Yes, I identified on the film all common and uncommon spectrum lines of the element magnesium. There was no other metallic element in the sample, not even the so-called "trace elements" usually detected in metallic samples.
Your report suggests that the metal in the sample was absolutely pure in the spectrographic sense, with a percentage of 100. Why did you not state this very interesting conclusion?
Because a pure metal in the spectrographic sense may still contain other possible constituents which could be present in your sample and still escape detection. The method as its limitations. Different compounds or states of combination of the same element, for instance, are not distinguishable by spectrographic analysis. Most of the nonmetallic elements are not detected by it the exceptions are very few. In in this particular case it could be a mixture of the elements found with any of its compounds, or a chemical combination with any of those nonmetallic elements a salt, for ample, despite the fact that the appearance of the sample suggests the element as being in its metallic form.
Will you detail the spectrographic plate for me?
Of course. Here [showing me the film] you can see five spectra. The spectrum corresponding to the sample analyzed is the first one, at the top of the film. It shows a number of spectrum lines with different strengths, but all of them belong to the same element they represent the spectrum of magnesium. The other four spectra were made for comparison purposes. The third is also a magnesium spectrum and corresponds to a chemically pure magnesium salt, CO3Mg. The remaining spectra are iron, Fe, comparison spectra
A photocopy of the Barbosa spectrographic plate was requested later and obtained.
(signed)
(signed)
|
(signed)
|
To confirm the results of the first investigation and to obtain a more accurate evaluation of the findings, I requested a second spectrographic analysis of the material. It was made on October 24, 1957. Another metallic chip from Sample 1 was put under the Hilger spectrograph by Elson Teixeira who for fifteen years had handled spectrochemical analysis at the Mineral Production Laboratory. His experience included more than fifty thousand spectrographic determinations. He had left the laboratory a few years earlier to enter business, but he still had permission to use the laboratory's facilities. He agreed to make a second spectrogram of the magnesium sample instead of doing a report on the analysis made by Dr. Barbosa. His problem was to determine whether or not the magnesium was of absolute purity in the spectrographic sense.
He used special technical procedures to control the many variables that might influence the results (such as atmospheric contaminants, dirty electrodes, etc.) Mr. Teixeira's analysis was translated verbatim. (Figure 2). He had also planned a "semiquantitative" spectrographic analysis to establish the percentages of any possible impurities not detected in the previous spectrogram of the magnesium sample. But his analysis confirmed the reported absence of impurities of any kind and therefore he felt that the "semiquantitative" test was obviously unnecessary.
The spectrographic film accompanying Teixeira's analysis was sent to me.
Incidentally, the two spectrographic analyses included in this report were not the only ones made of the magnesium samples. A third spectrographic study of the material was conducted by the military. The Brazilian Army had been informed about the case, and I was contacted by Major Roberto Caminha who requested and received a sample of the material on November 4, 1957. The military analysis was made at ITM (Military Institute of Technology), but I was not informed of the findings. It was intimated a complete investigation would be ordered by the Brazilian Army, but I was unable to confirm this information.
Another small fragment (the last piece of Sample 1) was given to Commander J. G. Brandao of the Brazilian Navy who contacted me a few months later. No information was obtained concerning the methods employed and the results in this investigation, but there are reasons to assume a spec trographic test (the fourth) was made at the Navy arsenal in Rio de Janeiro.
Since the spectrochemical analysis by Dr. Barbosa indicated the metal in the samples was pure in the spectrographic sense, other tests became necessary to correct the limitations of the spectrographic method and to investigate the possibility of nonmetallic impurities in the material. The remaining fragments of Sample I were sent to the Laboratory of Crystallography of the Geology and Mineralogy of the National Department of Internal Production for X ray diffraction studies. The director and chief chemist of this research institution, Dr. Elysiario Tavora Filho, is recognized for his pioneer works on crystallography since 1949, and is professor of mineralogy at the National Chemistry School. He is responsible for the results presented below. In my opinion his work is complete and flawless in every detail.
The X ray method of chemical identification was obviously indicated to complete the results obtained with the spectrographic analysis of the magnesium samples. The advantage of the procedure are that only small quantities of the material to be investigated (only a few Mg) are required, and that different compounds or states of combinations of the same elements are distinguishable since they posses different crystal structures. It is widely used for the identification of alloy phases. If more than one variety of crystal is present in any specimen, each will produce its spectrum independently (a very important fact to remember), and the pattern will consist of superimposed spectra with relative intensities depending on the relative amounts of the phases. Thus, the constitution of inorganic and organic systems, minerals and alloy systems can be determined with accuracy through X-ray crystallography. Besides, X-rays are also applied for chemical analysis through the use of X ray spectrometers that record the characteristic X ray emission lines, or absorption edges, of the sample. Favorable combinations of elements permit extreme sensitivity in the detection of small percentages of an element in a compound or mixture (independent of the state of chemical combination), and also permit fair precision in quantitative analysis.(Von Hevesy, G.: Chemical Analysis by X Rays and Its Application McGraw Hill, New York, 1932)
Because the precise results of X ray diffraction analysis, together with the advantages noted above, make it a sensitive method to determine the composition and structure of metals, it was decided to use this analytic procedure in the investigation of the magnesium samples. The conclusion that the metal was of absolute purity (in the spectrographic sense), with no detectable inclusions of other elements, was one all previous investigators hesitated to accept without confirmation by another method.
A preliminary identification of the samples by X ray spectrometry confirmed the previous report. The metal was really magnesium and appeared to be of unusual purity, with a percentage of about 100. Amazed by this truly incredible result Professor Filho repeated the spectrometric examination several times always with the same findings. He then decided to request a careful reexamination of the spectrographic plate to recheck the reported results of the spectrographic analysis. One of his assistants, Dr. Augusto Batista, was sent on that mission to the Mineral Production Laboratory. Informed about these unexpected developments, I was puzzled and failed to recognize the significance of Professor Filho's approach. He adopted a reserved attitude concerning the motivations for his decision, and I was unable to get any clue from Dr. Batista. As I was informed later, however, Professor Filho had realized the full implications of the reported absence of any impurity in the samples. The X ray diffraction diagram matched a standard diagram of high quality that was available for comparison, printed on a card from the Current X ray powder data file (and its accompanying index volume). That "standard" diffraction pattern had been produced, however, using the available ASTM standard of purity for magnesium (ASTM 4 0770), which is the spectrographic analysis still showed several impurities. The conclusion was that the magnesium in the samples would be purer than the ASTM "standard of purity" for that metal. It would be a truly incredible discovery, one that could not be accepted easily. Therefore a verification of the spectrographic analysis was ordered. When he saw the reported results confirmed, Professor Filho was probably inclined to reject the whole thing at first sight. But he had no choice. As a true scientist, he could not discard the hard, cold facts of the evidence obtained in the previous analysis. So he decided to use the most sensitive procedure available at his laboratory to settle the question, if possible. He decided to make a careful and complete study of the powder diffraction pattern of the mag nesium in the samples, using the powder method.
Professor Filho's Laboratory of Crystallography is equipped with the most elaborate and sensitive instruments for X ray diffractometry and spectrometry available anywhere. A powder camera of the Debye Scherrer Hull type was employed. A fine grained polycrystalline specimen of the magnesium sample was prepared. Its diffraction pattern was recorded on a special photographic film (of the cylindrical type) and that film was the object of careful examination. From the position of the lines on the film the so called "Debye rings" the spacing (d) of the corresponding atomic planes was determined. From the X ray picture, supplemented by other data, Filho determined space lattices, the spacing (d) already mentioned (interplanar distances) and the values of theta (bonding angle). The relative intensity of each line (or arc) on the film was also measured. The pattern obtained matched the diffraction pattern of the ASTM standard of purity of magnesium referred to above (ASTM 4 0770). All lines in the film were accounted for with the exception of six very faint ones. These did not correspond to the metal. They indicated that the sample contained inclusions of ail unknown crystalline substance, which was present in very small amounts.
Was it the impurity of impurities the chemists were attempting to detect since the first examination? The identification of this unknown material was the next step, for obvious reasons. But this task was going to be difficult because the unidentified component was not present in sufficient amount to give a characteristic diffraction diagram. In fact, the six reflections in the film which were accounted for were too weak to be used. A possible method to solve this problem was to expose different films for different lengths of time, thus making possible the measurement of strong intensities on one film and weak intensities on another. However, Filho decided on another approach.
The appearance of the "fragments" suggested that at some time they had been subjected to violent oxidation over all their surfaces, which were covered with a powdered material presumed to be magnesium oxide. This oxide, in Filho's opinion, might possibly be present within the body of the samples as the unidentified constituent. A plausible theory, and consistent with the claimed origin of the samples. The oxide formed on the surface of molten magnesium exposed to air would be present within the metal as a result of oxygen diffusion through the samples at ignition temperature. A microscopic examination made by Batista showed findings that appeared to support this theory. In fact, some of the small magnesium chips (taken from Sample 1) were covered with the powdered substance at points that corresponded to the surface of the original "fragments," and showed a few cracks and small fissures also filled with the same material. In these areas the crystalline metal was shot through with fissures containing that material too. On the other hand, it is true such areas were scattered and small, most of the samples showing only the crystalline pattern of pure metal. Besides, under microscopic examination, powdered specimens showed only a kind of crystal. They did not present any visible trace of the nonmetallic inclusions. Obviously, the mixture was not homogenous: the nonmetallic component was more abundant in areas close to the surface of the original sample; it might be present within the metallic mass, but in very small amounts, possibly in sufficient amount to explain the six unidentified lines on the film. As to the grain structure of the metal itself, Batista was almost sure the samples were fragments of a magnesium casting. Unfortunately, their appearance suggested they were not from the surface of the original casting, but came from within the metallic mass disrupted in the explosion; as a result, no information could be obtained on the thermal and mechanical treatment involved in the production of the casting. He also verified that the heat developed in the fragments when they were at ignition temperature had influenced physical and chemical properties at the surface of the molten metal, but apparently was too brief to produce gross melting or other recognizable changes in the grain structure. The accuracy of these observations was apparentry confirmed by the diffraction pattern for the magnesium in the samples.
These findings supported the hypothesis that magnesium oxide was the unknown component. Since the patterns of known materials can be used to identify the composition of an unknown constituent, the diffraction lines of magnesiurn oxide were studied. It was found the unidentified lines on the X ray film did not belong to that pattern. Therefore the composition of the dry, white powder on the surface of the samples should be determined too, and a second diffraction pattern was made, using this material. As a result, the non metallic powder was identified as magnesium hydroxide,(OH), plus magnesium in its metallic form. The hydroxide was obviously the unknown component already detected, for the unidentified lines on the first film corresponded with the diffraction pattern of this substance. No evidence was found concerning magnesium oxide, which was not present at least in the analyzed samples (from Sample 1). If a surface film of oxide was eventually formed while the molten fragments were falling through the air, or during the initial melting stage, it certainly was removed when the heated meta was cooling rapidly in the sea water. It is evident, on the other hand, the hydroxide in the samples was not a constit uent of the metal in its original form, appearing as an effect of oxidation in contact with water (the fall into the sea of the burning magnesium fragments, if the story of the samples origin is true).
The diffraction patterns recorded for magnesium and magnesium hydroxide were presented side by side in the photocopies of the original Filho films that were obtained.
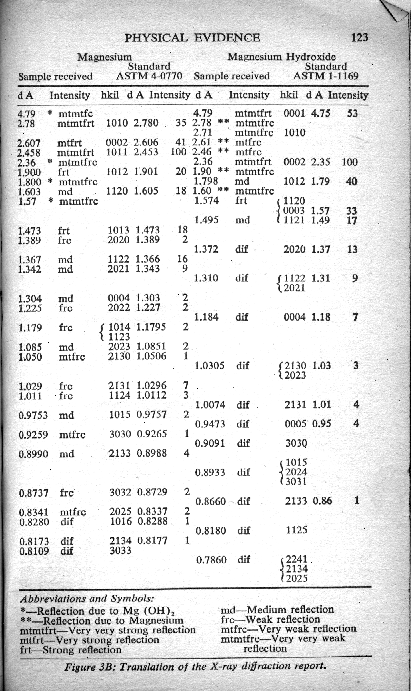
The X ray diffraction diagrams determined for each material, in comparison with the standard diagrams of the respective ASTM standards of purity, are presented in Figure 3A, which is a photocopy of Filho's original report on the X ray diffraction analyses of the magnesium samples.
For those who possess the technical background necessary for an interpretation of technical data presented in the Filho diagrams, a translation of his report is presented in Figure 3B.
Professor Filho's promised written statement on the possible origin, of the magnesium samples, in the light of the data obtained with X ray diffraction analyses, was not received due to the unexpected results be found, Filho decided only numerical data should be released : written statements or conclusions of any kind could not be issued because he didn't want to discuss certain problems connected with the origin of the samples.
The relative density of the magnesium samples (expressed in terms of water at 4 degrees C.) was measured at the Laboratory of Crystallography by Dr. Batista. The method used was the classical procedure involving two weighings, the relative density of the metal being determined by a simple formula (the weight of the specimen in the air divided by the loss of weight when suspended in water). A Jolly balance of the type used by mineralogists was employed.
Previous studies suggested large pieces of the metal should got be used. Their surfaces were covered with magnesium hydroxide, a denser material; areas within the crystalline metal with inclusions of this material were also observed. One of the two remaining "fragments" (Sample 2), for example, evidently contained more hydroxide inclusions than the other one (Sample 3), but the appearance of both samples indicated their relative densities would not correspond to the values predicted for magnesium.
In fact, they would represent only the average densities of samples containing unknown amounts of a denser material. To solve the problem, Batista selected a small metallic chip taken from the center of the divided "fragment" (Sample 1) for the density determination. This specimen was carefully polished until the silvery white surface of pure magnesium showed no trace of hydroxide under microscopic examination. Such a sample should have a density of about 1.741, but a significantly higher density was found the carefully measured density of this magnesium sample was 1.866. The procedure was repeated three times with a microbalance, and the same value was found each time.
How could this discrepancy be explained? Three possibilities had to be considered:
Interpretation of the available data suggested the second possibility was the most plausible explanation. It was possible a small inclusion of hydroxide was still present in the specimen (rendered plausible by the X ray diffraction analysis). The density measurements gave no ground for reliance on an unusual isotopic ratio. On the other hand, the powder diffraction pattern showed hydroxide was mixed with the pure metal in very small amounts too small, apparently, to explain the high density found. This discrepancy can be resolved only with careful determinations, using several metallic chips taken from the samples. It is evident the hydroxide cannot be evenly distributed through the whole metallic mass, and tests with different samples will show different densities. If any of the density measurements corresponds to the expected value for terrestrial magnesium, the problem is solved. But if any discrepancy remains even a small one then a mass spectrographic analysis is necessary to study the isotopic constitution of the magnesium samples. The reasons will be discussed in another section of this report.
The magnesium in the samples analyzed, which was absolutely pure in the spectrographic sense, represents something outside the range of present day technological development in earth science. In fact, the metal was of such fantastic purity that even to see it symbolized on paper is unbelievable. Even the infinitesimal quantities of "trace elements" usually detected by spectrographic analysis traces so small they could not possibly be detected by any other analytical method were not found. Thus, the magnesium in the samples was absolutely pure in the spectrographic sense with a percentage of 100. X ray spectrometry and X ray diffractometry by the powder method confirmed the results of the spectrographic analyses the metal was pure magnesium
Again, no impurity was detected to introduce irregularities, in the crystal lattice. The presence of any impurity of any interstitial atoms would change the regularity of the crystal lattice, thus causing crystal imperfections that would be revealed by the X ray method. Therefore, on the basis of the chemical analyses the conclusion was that the magnesium in the samples was of absolute purity, in the sense that any other possible constituents which could be present would lie present in such an infinitesimal amount as to be beyond the reach of any known method of chemical analysis.
We know very little about metals completely free of impurities and imperfections, simply because they are never found in nature and, in most cases, cannot be prepared in the laboratory. It is not too difficult to refine a metal to 99,99% purity (which means there is something else besides the metal to the extent of 1 part in 10000), but once beyond this point the going gets rough. For every 9 we tack on after the decimal point following the first two 9s, the cost increases tenfold, sometimes a hundredfold. This is so because involved, delicate and time consuming crystallization operations are required so that the final product becomes more precious than gold.
In the study of the properties of absolutely pure metals the first problem is to secure them. As a matter of fact,
the task seemed hopeless for any metal until eight years ago when the American metallurgist Walter Pfann invented
the zone refining process, which promises to be one of the outstanding developments in the story of the
metallurgist's efforts to produce "super pure" metals. With this method it is possible to produce germanium and
molybdenum (also iron and titanium, according to some sources of information) of almost absolute purity. However,
even with this process, everything has to be done piecemeal: metals cannot be purified continuously. This one great
drawback to the large scale production of pure metals seems now to have been overcome by a new development announced
by Dr. Pfann five years ago. His new invention, based on the zone refining method and called continuous
multistage zone refining
, will make it possible to obtain pure metal in a continuous flow.
Such is the situation concerning the latest developments in the field of "super pure" metals. A few can already be refined to approach absolute purity, but the problem still remains unsolved for the other metals, because of technical difficulties not yet solved. Magnesium is included in this latter group. In other words, to produce magnesium of absolute purity is still an impossible task. Getting rid of the last bit of impurity is impossible, even in the laboratory. If this postulate is correct the magnesium in the samples analyzed could not have been produced here or recovered from the explosion of a man made missile or vehicle. It is, then, of interest to discuss the matter further for direct and indirect support of the postulate.
Magnesium occurs abundantly on earth, but never in the pure state always in combination. The meteorites. (almost entirely composed of common silicates and nickel iron) reaching earth may contain magnesium, but always in combination (magnesium oxides, silicates, etc.), never in the pure state. The production of metallic magnesium requires special extraction and refining methods, the most widely used being he process of electrolytic reduction of magnesium chloride derived from sea water, natural brines, potash waste liquors, dolomite and magnesite. Thermal reduction processes are also available; they are of two types one using carbon (the Hansgirg process), the other using ferrosilicon (the Pidgeon process) for the reduction of magnesium oxide derived from magnesite, dolomite or sea water.
Refined commercial magnesium of a purity of 99.8% Mg (pure magnesium, ASTM: B 92 45) can be produced by any of these methods in the form of ingots, powder, ribbon,, wire and extruded and rolled strips. Impurities such as iron, nickel and copper have definite tolerance limits because the quantity and state of these impurities determine the resistance of the metal to corrosion. Some elements are not harmful in large proportions, but others are detrimental even when present in minute amounts. Calcium is usually present in very small quantities, chiefly in solid solution; if present in amounts greater than approximately 0.1%, calcium occurs as Mg2Ca. It is not harmful, and in some magnesium alloys (MI and AZ31X), it is added to improve such characteristics as the grain size of the ingot, rolling properties and ductility. Excessive amounts, however, are considered detrimental to welding characteristics in some alloys.
In common with aluminum and many other metals, magnesium is not used commercially without alloying. Manganese, zinc, zirconium and aluminum are the chief alloying components of magnesium alloys. Magnesium cerium and magnesium thorium alloys are more recent developments.
Silicon is the impurity usually picked up in ordinary foundry operations and occurs generally as Mg2Si. If present in amounts of 0.5% or more, it changes the regularity in the crystal lattice, causing defects in the magnesium crystals.
The presence of even a few hundredths per cent of manganese greatly increases the tolerance limit for iron (which is 0.017%, for pure magnesium) and also for nickel.
Composition limits for commercially pure magnesium (ASTM B 92 45 for ingot and stick) are: Pure magnesium sheet, wire extrusions, ribbon, and ingot and stick for remelting: 99,80% Mg min.; impurities (max.), 0,02 % Cu, 0,001 %, Ni, 0,20 % total of Al, Cu, Fe, Mn, Ni and Si. Powder, grade C: 96 % Mg min.; impurities (max.), metallic Fe 0,05 %, insoluble residue 0,25 %, Si 0,10 %, grease and oil 0,020 %, alloyed iron and aluminum as oxides 0,40 %.(Townsend, R. A.: Properties of Magnesium and Magnesium 41loys. ASTM Metals Handbook, Cleveland, 1954.)
It is evident the quantities of impurities found in commercially pure magnesium vary according to sources of production and methods employed. In any case, however, they are always present, even in the composition of the purest commercial magnesium available. It can be concluded that no commercially pure magnesium exists with a composition at all like that of the samples analyzed.
To complete the investigation on this important point, I decided to test the accuracy of the spectrograph in detecting these impurities in commercially pure magnesium. But typical samples of this metal were not available; the metal is not produced in Brazil, except in powdery or granular form.
As an alternative, tests were made using chempur magnesium salts and samples of commercially pure tin and lead. All elements whose presence was predicted in each sample, even the so called "trace" elements, were detected in spectrograms made with the same Hilger spectrograph used for the magnesium samples. The Teixeira investigation confirmed the high precision and accuracy of the instrument. It also eliminated the possibility of other constituents which could escape detection. On the basis of these studies, it is evident the person who supplied the samples could not have obtained them from any available source.
The ASTM standard of purity for magnesium (ASTM 40770) shows in spectrographic analysis the following impurities: Ca 0.1%; and traces of Al, Cu, Fe and Si (Swanson and Tatge: J.C. Fell Reports. NBS, 1951.) This is the purest magnesium that can be produced by present day processing methods and refining technologies of terrestrial metallurgy. The conclusion is that the magnesium in the samples analyzed, which was absolutely pure in the spectrographic sense, is better in quality than the purest magnesium refined on this planet and represents something outside the range of present day technological developments in earth science.
On the basis of this. evidence, it is highly probable the metallic chunks picked up on the beach near Ubatuba, in Sao Paulo, Brazil, are extraterrestrial in origin. This is indeed an extremely important and almost incredible conclu Sion. But on the basis of the findings of these chemical analyses there is no other alternative. As staggering as the implications may be, this appears to be the only acceptable explanation. Therefore, the magnesium samples analyzed must represent "physical evidence" of the reality and extraterrestrial origin of a UFO destroyed in an explosion over the Ubatuba region. They are, in fact, "fragments" of an extraterrestrial vehicle which met with disaster in the earth's atmosphere, as reported by human beings who witnessed the catastrophe. The gratifying aspect of this case, however, is that we do not have to depend on the testimony of witnesses to establish the reality of the incident, for the most advanced laboratory tests indicate the fragments recovered could not have been produced through the application of any known terrestrial techniques.
Further investigation of the incident will be necessary, of course, but only to complete the information already obtained and, if possible, to obtain more samples of the material for additional examinations. I had in my possession three fragments of the "flying disc." Sample I was used for the chemical analysis made in Brazil. Sample 2 was divided and a large piece, roughly a rectangular prism approximately 1.2 x 0.7 x 0.7 centimeters, was sent to Coral E. Lorenzen, director of the Aerial Phenomena Research Organization in Alamogordo, New Mexico. This sample can be used for other analyses, if necessary. However, if other tests are needed for a critical evaluation of the Brazilian analyses, special precautions must be taken from a technical viewpoint. The reasons are obvious. It is far more difficult to prove the "absolute purity" of a metallic sample than to show the presence of, "impurities." Thus, spectroscopic tests cannot be accepted because they are based on the visual impression of the technician conducting the test they cannot be rechecked by other observers. Spectrographic tests done in a routine manner, using standard electrodes and making an exposure of a fixed length, cannot be accepted either. A spectrum must be run on the electrodes for reference, and possible impurities in the carbon rods used as electrodes (such as traces of Mn, Fe, Si and Ti) sometimes appear as contaminants; they cannot be subtracted out on the basis of a standard assumption of purity, i.e., assuming that all electrodes have the same impurity content. Many variables have to be controlled, such as atmospheric contaminants, dirty electrodes, use of different electrodes, use of different excitations techniques, etc. These are some of the corrective measures to avoid mistakes, especially in this case, in which a claim of "absolute purity" was established on the basis of chemical examinations. We need a true scientific research, not a routine examination of the samples. Incidentally, Sample 2 was not analyzed in Brazil, but there is no logical reason to suspect it is less pure than the other the material is similar in appearance and came from the same object.
Density measurements of magnesium chips from Sample 2 must be made to resolve the discrepancy represented by the high density found in previous tests. If any discrepancy still remains a mass spectrographic analysis is indicated, to study the isotopic constitution of the magnesium in the samples.
|
Magnesium has five isotopes, but only three are stable; the two others are unstable, having a very short half life. It is a striking fact that, with few exceptions, the relative abundance of the isotopes for each element is the same once and for all. The exceptions are the elements Pb, He, C, 0, N and S. Apart from these minor exceptions, in the early geological period in which minerals were formed a certain isotopic constitution appears to have prevailed over the material now accessible to our investigation. (Figure 4 shows the isotopic abundance of terrestrial magnesium.)
A higher density might indicate a different isotopic constitution in the magnesium of the samples, if the possibility of a small inclusion of hydroxide is excluded. after a careful evaluation. An unusual isotopic distribution probably a preeminence of the heavier isotopes 25 and 26 would be absolute proof of the extraterrestrial origin of the metal, in my opinion.
Are the relative abundances of the isotopes of each element characteristic only for the earth? We don't know. The little material we possess derived from the investigation of meteorites (which, presumably, are members of our solar system, too), shows they present the same relative abundance as the elements known in the laboratory. if this could be proved for all the planets in our solar system, and for planets in other solar systems, the possibility of metals with unusual isotopic constitution could not be discussed. With our present knowledge, however, we must be prepared to consider it in this case at least as an interesting theoretical possibility. For technical reasons such a study was not made in Brazil. A mass spectrographic analysis may solve the problem. Or perhaps the isotopes can be identified by their microwave spectra; if so, microwave spectroscopy might serve as a quick means of measuring how much of what kind of isotope is present, to at least show if the magnesium is a naturally or artificially mixed sample.
The available evidence seems valid enough to establish that the magnesium fragments were recovered from the explosion of an aerial object of artificial origin; that this disc shaped object was not a man made missile, an artificial satellite or a remote controlled device but an aerial machine of extraterrestrial origin. The question of the place, means and purpose of the original fabrication cannot be solved with the evidence at hand. Yet a few deductions can be attempted to explain the mystery of the UFO's sudden explosion and some other important issues of the Ubatuba incident.
In my opinion, these electromagnetic fields suggest another explanation making unnecessary the existence of an artificial gravity field around the UFOs. Recent developments in the field of hydromagnetics seem to indicate that the heating effect on the surface of a rocket or missile can be avoided by using magnetic fields. The possibility was discussed by Dr. W. F. Hilton, chief aerodynamicist of the ArmstrongWhitworth Aircraft Company in England.
From a study of thermonuclear work on the "pinch effect," we decided to try the effect of magnetic fields on the hot flow from our company's shock tube. The basis of this interaction is the very great heating of air behind the shock wave from the front of the vehicle. This heating causes the air to become partially ionized into electrically charged particles, and these particles in rapid motion past the vehicle have the nature of an electric current. They are, therefore, susceptible to deflection by means of a magnet. So far our results have been very encouraging, and we have been able to provide quite definite deviations with a small electromagnet powered by a 12 volt battery. Whether this effect will lead to a practical contribution to reentry remains to be established.(UFO Investigator, 1: 1, August September 1958)
In a recent report to the American Rocket Society, Princeton University physicist Dr. Russell M. Kulsrud stated that the new field of "hydromagnetics" (formerly called magnetohydrodynamics) might help solve the missile reentry problem(UFO Investigator, 1: 8, December 1958.). In nuclear fusion devices (H bombs) for instance) magnetic fields are used to keep electrified gases away from the walls of a container long enough for the nuclear reaction to take place. The same principle, he said, might be used to deflect hot gases generated by devices plunging into the atmosphere. Dr. Kulsrud, who is working on the Princeton plasma physics Project Matterhorn, also said that the sciencefiction concept of using invisible "force fields" to repel incoming objects was becoming a reality in hydromagnetics.
Hydromagnetics deals with the reaction of "plasma" fluids to, high magnetic fields strong enough to control charged particles moving in a "plasma" and smaller electric fields. In the "pinch effect," the flow of an electric current through a gas generates a strong magnetic field which at once contains the gas and brings it up to high temperature by compressing (that is, pinching) it. In my opinion, ionization and magnetism combine to produce a hydromagnetic effect on the air in rapid motion around a fast moving UFO i.e., energized ions, atoms (or positively charged nuclei) and free electrons in the air are contained in a magnetic field. Thus contained in the magnetic field, the ionized air will not touch the surface of the object. In the particular UFO case a magnetic field produced independently of the electric current that heats the gas in the pinch effect was necessary (a pincheffect current was not needed because the very great heating of air behind the shock wave already made it partially ionized into electrically charged particles). This could be obtained by an externally applied, rapidly pulsating magnetic field. The charged particles moving across the field would experience a deflecting force and proceed to gyrate in circles about the lines of magnetic force. An electric current would flow through the air along the magnetic surfaces. The power dissipated by the resistance of the gas would go into ionizing and heating the air, as well as into producing some ultraviolet and visible radiation. This might be called "ohmic heating." (it is the ohmic resistance of the gas that generates the heat on passage of the current). Unlike a pinch effect current , this ohmic heating current will not produce any contraction or compression of the ionized gas. As a result, the strong magnetic field around the UFO would hold the gas firmly in place and almost constant in volume. Such a magnetic field must be "force free," i.e., capable of maintaining its form through a balance of purely magnetic forces. It is already proved that a force free magnetic field is possible in a toroidal shape.
In fact, there are magnetic fields (according to the German astrophysicists A. Schluter and R. Lust) that possess certain field configurations which are "force free" in the sense they do not tend to expand or distort their shape. If we assume a set of wires wound into a metallic object in such a manner as to produce a three dimensional magnetic field, the object would have a longitudinal field into the coil (inside its walls), seeking to expand, and a circular field running around it, seeking to contract. In short, these fields would balance so that no inward or outward force exists. The trouble would come at the end of the system, for the compensation would break down there and the force free configuration consequently would be disturbed.
A way out is suggested by the torus or doughnut a system without end. It seems obvious that in a disc or saucer shaped object the "coil" bends in a circle to form a closed but endless system. In the resulting toroidal or doughnut shaped magnetic field the lines of force become circles and the path of each charged particle is a helix. Yet such a toroidal field is not stable enough, due to the effect of particle drift. In fact, as a result of curvature, the strength of the magnetic field is greater near the inside than it is near the outside. This inhomogeneity of the field alters the helical path of charged particles. The result is that the charged particles drift across the field, the positively charged ones collecting at the top of the tubular field and the electrons at the bottom. This drift is bad enough in itself, but its indirect effect will be catastrophic. The resulting separation of electric charges produces a large electric field which will completely disrupt the particle paths, throwing the entire gas into the surface of the UFO due to the fact that a steady electric field imposed across a magnetic field produces no current all in a fully ionized gas, but drives the gas particles indirection at right angles to both the electric and magnetic fields. The UFO would be destroyed in the process.
There is a simple solution for this drift of charged particles across a toroidal magnetic field. By one means or another the magnetic field can be twisted around its circular axis, giving the lines of force a helical form like the strands of a rope. In this twisted toroidal field the effect of particle drift is much reduced. Oppositely charged particles will still show some tendency to drift apart, with an accompanying separation of charges, but now the charges can leak back along the lines of force. Any difference in electric charge along a line of force will thus be eliminated, and a steady confinement of the ionized air now becomes possible. The necessary twist can be imposed on a toroidal field in a number of ways. Passing an electric current along the lines of magnetic force in a torus will do it, but such a current would require pulsing every few seconds. Another way is the method in which the toroidal field is twisted by interaction with an additional transverse magnetic field (generated by a set of helical windings in which the current flows in opposite directions in adjacent groups of wires).
It is my opinion hydromagnetics would explain the UFO's apparent immunity to air friction and suggest a possible power source. It is postulated that ionization and magnetism produce a hydromagnetic effect on the air surrounding a highspeed UFO which avoids any contact between the gas and the object's surface. There is, first, the very great heating of air behind the shock wave from the front of the vehicle, causing the air to become partially ionized into electrically charged particles, and these particles in rapid motion past the vehicle have the nature of an electric current. The interaction of an independently produced magnetic field, possibly created by an externally applied, rapidly pulsating magnetic field, holds the electrified particles in circular orbits. This force free field is probably a twisted toroidal magnetic held (or a special field configuration with similar properties). The deflected particles are kept away from the UFO's surface; the charged particles and atoms collide only with each other, and the plasma becomes fully ionized. A circular electric current flows into the doughnut shaped plasmoid thus formed around the object. This plasmoid acts as a "cushion" of high magnetic field pressure between the object and the surrounding atmosphere (like an invisible "force field"), but does not touch the surface of the UFO which moves inside of it in a kind of aerodynamical vacuum." The strong force free, rotating magnetic field holds the plasmoid firmly in place and constantly (or almost so) in volume around the object. The ohmic heating current (unlike the pinch effect current of thermonuclear experiments) produces no contraction or compression of the ionized gas confined in the externally applied magnetic field around the UFO. It is obvious, however, that the air does not remain steady and motionless during ohmic heating; in fact, the ionized gas is expected to develop violent activity during the process. The object's own motion, plus the effects of electric and magnetic forces involved, introduce complications which make the activity quite different from ordinary turbulence. Ultraviolet and visible radiation certainly are produced as a side effect. In addition, the "cooperative activity" of charged particles in the heated and ionized gas can produce many other effects, some of them not yet understood such as the production of radio noise bursts similar to those observed from the sun. Disturbances of the hydromagnetic type can also be expected, as well as the appearance of "runaway" electrons that no longer can be confined and strike the object, producing intense X rays (this tendency is probably reduced with a twisted field).
It seems reasonable to expect that a high magnetic field strong enough to form and maintain a plasmoid around a fast moving UFO through a balance of purely magnetic actions would protect UFOs against their friction at any speed, thus avoiding any heating effect. Also, these effects correspond with many of the unexplained phenomena reported in connection with UFOs. But magnetic fields are, of course, invisible and lines of force are purely imaginary constructs. How can we "see" them on UFOs? A way out is suggested by the Zeeman effect, which certainly would be detected in the spectrum of light emitted from UFOs at night. Available empirical evidence suggests careful research of the theory. Fields of the magnitude we have been discussing are probably strong enough to dominate the motion of charged particles within atoms, to cause some crystals to contract, to make a conducting metal extremely resistant to electrical current or opaque to infrared radiation, and perhaps to produce a measurable "gravity field" effect, too.
In the case of a UFO moving at low speed, or halted in mid air, the' heating of air particles is possibly not enough to generate a plasmoid around it only the magnetic field would exist. If necessary, however, magnetically confined plasmas might be generated by a rotating part in the object (a spinning ring, for instance), by rotation of the object itself, or with the help of special "plasma jets" on the object firing doughtnut shaped bursts of plasma. In the near vacuum outside the atmosphere these plasma jets also ,night operate as a possible propulsion source. On the other hand, it seems evident that if the object is moving at high speed in the dense lower levels of the atmosphere, the sudden collapse of the force free field and plasmoid would result in its thermal disintegration in a matter of seconds. The mechanism similar to the one discussed in connection with Plantier's theory, could certainly explain the mystery of the sudden explosion which destroyed the Ubatuba UFO.
(6) We are beginning to probe the new frontier of the socalled "thermal barrier" as our planes approach thermantic (Mach 3 to Mach 4) and superthermantic (Mach 4 to Mach 8) speeds. There is also the missile and satellite reentry problem. In the thermantic region (1325 to 2650 mph), stagnation temperatures (air's original temperature plus the heating caused by friction with the moving surface of a plane) range from 250' to 1500' F., varying from 1200" to 6300' F. or more in the superthermantic region. However, the picture is much less severe in the relation to equilibrium temperatures (those in the metal on the surface of the airplane). In the thermantic region, for instance, they get up to only 900' F. but this is still heat, and plenty of it). At superthermantic speeds the problem becomes far more difficult. Tomorrow's airplane may glow red and give off enough heat to heat four hundred average sized homes when reaching its equilibrium temperature at 180,000 feet at a speed of Mach 8. To solve the problems involved we are making an endless search for better and better heat resistant materials and cooling systems.
On the other hand, the available evidence suggests the Problem of the "thermal barrier" was solved by the intelligences behind the UFOs. These unconventional aerial objects can move across the earth's atmosphere at velocities between Mach 4 and Mach 8 or more, with apparent impunity to the heating produced by friction with air molecules Are they made with heat resistant material better than Pyroceran of Inconel x? This is apparently the obvious explanation despite the fact the extremely high speeds reported - certain cases would be enough to burn up even the best heat resistant material in the universe. Cooling systems are useless if the speeds are high enough.
The physical evidence in the Ubatuba incident provides a different answer for the question. It indicates clearly materials of high heat resistance are not the key to the "thermal barrier" problem. It is obvious an object made of magnesium (a metal of low heat resistance) could never stand the overheating at the unbelievable speed it was moving when first seen over the sea. The magnesium shell would lose its mechanical strength quickly and burn in a few seconds even at speeds far below the one reported. Yet the Ubatuba "flying disc" did not show any sign of overheating at any time before the explosion, despite its enormous speed. This is a very important point. As no trace of any protective coating was detected in the recovered fragments, it seems evident something invisible existed around that UFO to protect its magnesium shell against air friction. When that protection disappeared, thermal disintegration within a few seconds was the observed result.
Whether or not that something protecting the UFO against overheating at high speeds was an artificially thickened and controlled "boundary layer" (Plantier's theory) or a hydromagnetic effect producing a kind of "aerodynamic vacuum" (my hypothesis) remains to be established. At any rate, the conclusion is that our present approach to the problem should be carefully reevaluated. Our endless search for better and better heat resistant materials and cooling systems may show good results for some time yet, but it will not win this new frontier for us. A different approach should be tried, for it seems that more practical and efficient solutions can be found. On the basis of the evidence available on the Ubatuba incident, it is my opinion the key to the problem is just before our eyes every time a UFO is sighted.
Thus concludes Dr. Fontes's report. Only a few additional facts are required to bring it up to date.
Soon after receiving the samples from Dr. Fontes, APRO submitted a portion of a sample to an Air Force spectrographic lab for analysis. An "emission spec" was requested,The following day the emission spectrograph operator reported that he had accidentally burned the entire sample without obtaining an, exposed plate. He requested another sample APRO declined.
Our next venture fared little better. A piece of Sample was submitted to an Atomic Energy Commission labora tory. A density test was performed which involved creat ing a solution in which chips of the metal would neither float nor sink. This solution (a mixture of bromo benzine and brainstorm) was found to have a density of 1.7513 grams per cc., a little high but near normal for terrestrial magnesium (1.74 grams per cc.). The experts concluded that this small deviation was the result of a small inclusion of oxide in the chips and was insufficient grounds for belief in an unusual isotopic ratio, and that "the sensitivity of mass spectrographic determinations is such as to make such an examination completely unprofitable." A technician (who requested that his name be withheld) ran an emission spectrograph test which showed the presence of several trace elements, as follows: iron between .01 and .1%; silicon between .01 and .1%; aluminum between .01 and .1%; calcium between .0001 and .001%; copper between .0001 and .001%.
The instrument used was an Applied Research Laboratories two meter grating spectrograph with a dispersion of five
angstroms per cc. The technique used was the standard "semiquantitative" method prescribed for a magnesium matrix by
Harvey, using standard electrodes. The resulting film returned with the report showed five irrelevant spectra, the
magnesium spectrum and an iron spectrum for comparison. There was no separate spectrum of the electrodes, and it was
not possible to determine whether the detected impurities ere in the electrodes or in the sample. The impurities,
however, are those normally found in standard carbon electrodes. The complete test was, in Texeira's opinion, completely
valueless from a scientific standpoint
. A metallographer who examined the remaining portion of Sample 2 came to
the conclusion the sample was a portion of a casting which had not been worked mechanically since it had originally
"frozen" from the molten metal; the experience it passed through, which led to the oxidation noted, apparently having
been too brief to allow gross melting or other recognizable changes in the grain structure.
One thing seemed clear we were not likely to obtain satisfactory results simply by sending out samples and having faith; furthermore, our supply of the metal was dwindling alarmingly. After due deliberation, the staff decided that an attempt should be made to have the metal examined by a qualified laboratory with APRO and USAF advisors participating. This seemed the best way to insure no important aspect of the problem was overlooked. Accordingly, a letter was addressed to ATIC, with a copy to the press to insure prompt attention but to no avail. We received only a routine request to forward the purported material to ATIC at the Wright Air Development Center. We then attempted to establish liaison with ATIC, but they declined to answer our letter. We could only conclude that the USAF was not interested in any real answers, or already had obtained full details (and possibly samples of the metal) through classified channels. Our correspondence with the Air Force had one satisfying result, however. The resulting UPI news story brought the matter to public attention in Brazil. As a result all aspects du rapport du Dr. Fontès were verified in press and TV interviews with the principals.
The identity of the witnesses to the original incident remains unknown. In an attempt to locate them, Dr. Fontes and Joao Martins canvassed the beach area in the neighborhood of Ubatuba. Eventually they located a fisherman who remembered a group of vacationers from an inland town who told of the incident and displayed pieces of a gray substance to support their story. He could remember nothing else of any value except they were excited and talked eagerly of their experience. This information might only serve to deepen the mystery, except for this fact: During 1958 when Dr. Fontes was in the midst of his investigation of the strange metal he was visited by two members of a Brazilian intelligence agency. These two individuals at first made veiled threats as to what might happen to him if he continued his inquiry into matters that "did not concern him." When it became apparent that Fontes could not be coerced into silence, they appealed to his "better judgment" to cooperate with them and turn all his notes and the strange metal over to them.
It is my opinion that the original witnesses may have reported their experience to some official agency and that they thus lost their metal samples and were encouraged into silence. Another conclusion may be that official agencies learned of the incident in the same way Dr. Fontes did and contacted the witnesses through Mr. Sued. One researcher questioned the validity of the case on the basis of the lack of witnesses, and also claimed the British are able to produce pure magnesium. Inasmuch as names of scientists and laboratories supposedly involved have not been forthcoming, APRO feels the burden of proof is on the doubter, and to prove his case he must produce samples of 100% pure magnesium manufactured prior to September 1957.